Enter Forcyte Bio:
Cell function is the epicenter of disease and its treatment, and the ultimate goal of all therapeutics is to affect cell function. At Forcyte, our approach is to confront cell function at the outset of drug discovery, at the earliest stages to dramatically improve the odds of successful translation.
Our philosophy is to follow empirical causality over statistical association. To prioritize dynamic cellular function over static cellular structure. To laser-focus on specific fundamental disease mechanisms over broad biological profiles. To systematize biology rather than fear its complexity. At Forcyte, we are a function-first drug discovery company.
Our particular focus is on the physicality of disease. We understand that biology isn’t merely a realm of biochemical reactions and electrical impulses, but also a world governed by cellular mechanical cell function, or mechanobiology. Recognizing this, Forcyte Bio was founded on the conviction that we must explore, understand, and leverage this overlooked yet essential facet of biology in our pursuit of improving human health. By pioneering high-throughput drug screening technologies that assess the mechanical function of human cells, we aim illuminate this dark corner of the biological stage. The curtain is rising on the new era of mechanobiology and Forcyte Bio is leading the way.
The Body Is (Also) a Mechanical System
In the complex symphony of life, where genetic, biochemical and electrical systems often take center stage, a pivotal performer remains in the shadows: our cells’ mechanical systems.
Picture a grand ballet where cells are the dancers, gracefully moving, contracting, and responding to a multitude of physical cues. This dance is not merely an accompaniment to the music of life, but rather forms its very backbone. Cells, like the dancers, perform individually and as ensembles or tissues to faithfully recite their rehearsed routine, their physical function (in-step and precisely as it was choreographed) to ensure the show will go on.

Cardiac contraction, wound healing, the seemingly simple act of taking a breath, focusing our eyes, childbirth, even the division of cells all partake in this intricate choreography, and depend on correctly operating cell mechanical functions. Our cells move, change shape, tug, pull, and squeeze in a highly coordinated interplay of biology, chemistry and physics. Indeed, it’s this physicality of life, our human ‘mechanobiology,’ which is the crucial interfacial layer between our biology and the physical world in which we live.
But what happens when that coordination falters, when our cells stumble, tire, or miss a beat, or a forget their routine altogether? What happens when our cellular mechanical systems fail?
When the Ballet Goes Awry: Dysfunctional Mechanobiology leads to Disease
A misstep in this cellular ballet can result in an array of diseases, all underpinned by a failure in maintaining proper mechanical functionality within and by our cells and tissue. Cardiovascular conditions like heart failure and hypertension arise when heart cells lose their rhythm, failing to contract and relax as required, or dancing off-beat. Asthma, a respiratory condition, can be traced back to smooth muscle cells lining our airways contracting too tightly and crowding too closely.
Fibrotic conditions, such as pulmonary fibrosis or liver cirrhosis, occur when fibroblasts, the cells responsible for forming extracellular matrix and collagen, become over-zealous performers. In an uncontrolled dance, they squeeze too hard, stiffening their surrounding tissue and disrupting the normal function of organs.
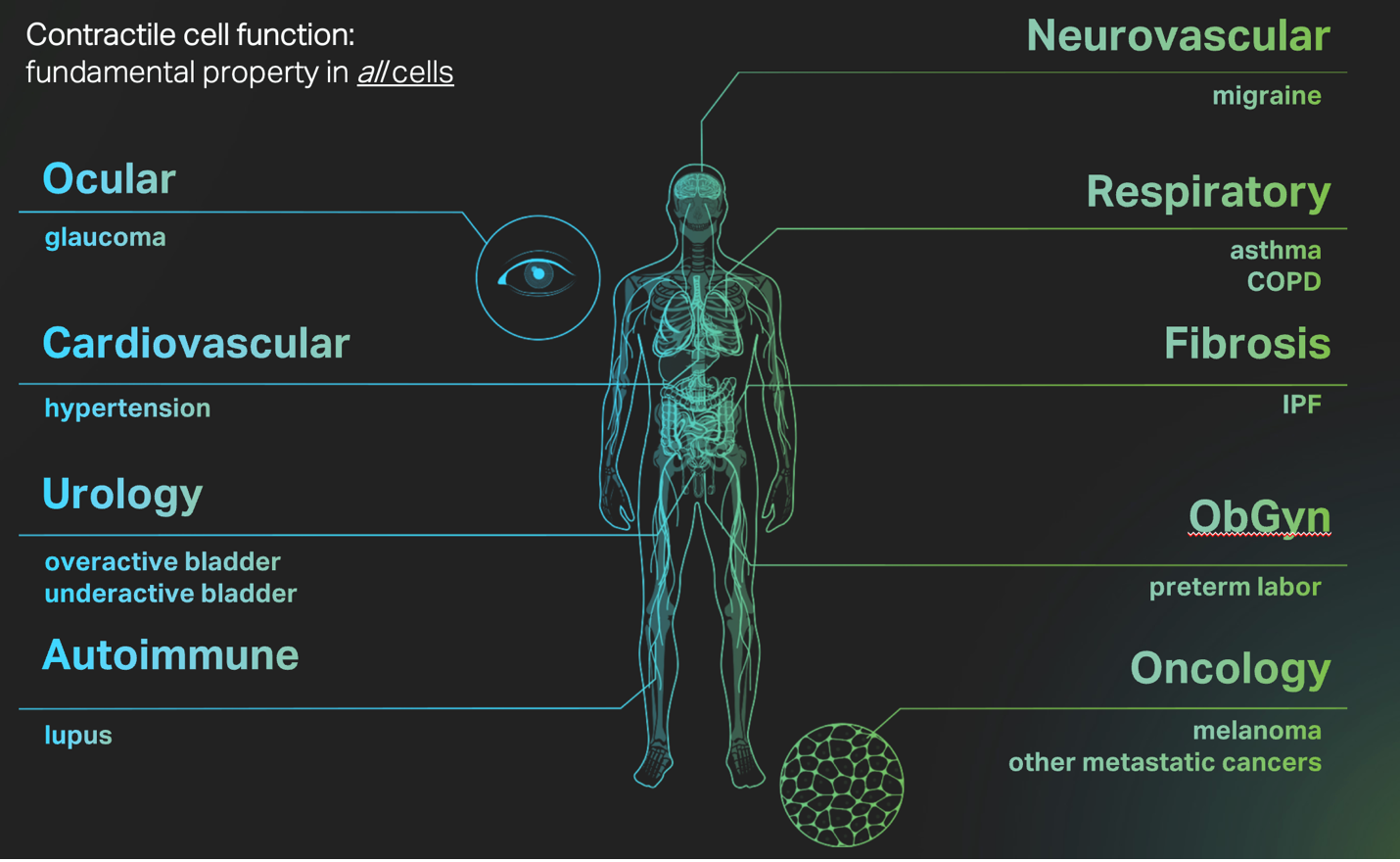
The list of pathologies that result when mechanobiology goes awry covers most, if not all, therapeutics areas, highlighting the ubiquity and centrality of this fundamental area of human biology.
As the visionary founding director of the Wyss Institute for Biologically Inspired Engineering at Harvard University (and Forcyte SAB member) Don Ingber stated in his 2003 review paper on mechanobiology:
The current focus of medicine on molecular genetics ignores the physical basis of disease even though many of the problems that lead to pain and morbidity, and bring patients to the doctor’s office, result from changes in tissue structure or mechanics.
and:
Re‐evaluation of human pathophysiology in this context reveals that a wide range of diseases included within virtually all fields of medicine and surgery share a common feature: their etiology or clinical presentation results from abnormal mechanotransduction.
Despite this, he voices the concern that:
…in the current genome euphoria, there appears to be no place for `physicality’.
Indeed, Dr. Ingber’s now 20-year old concern is well-founded; the practice of only pursuing so-called “genetically verified” targets (i.e. targets that have some human genetic evidence of disease association) by many large pharma companies has left the much to be desired in terms of unmet clinical needs in chronic disease areas, particularly complex and multifactorial diseases lacking a strong genetic component such as fibrosis, COPD, and under-active bladder (all, incidentally, diseases of mechanobiology). For this reason, Forcyte Bio places cellular function first, focusing on the clear and real-time basis of these complex diseases: their physicality and underlying mechanobiology.
Chasing the Rhythm: An Intuitive Journey to Identifying Mechanotherapeutics
We believe the path to identifying treatments to these mechanical diseases is as intuitive as understanding a dance — by focusing directly on its choreography. In mechanobiology, its the contraction and relaxation of the cells that form the core of its performance. And, just as you can’t evaluate a ballet without actually observing it, the same applies to cellular mechanobiology.
To truly discern the intricate workings of our cellular ballet, we must immerse ourselves in its performance, and build tools to directly quantifying mechanical cell function as it unfolds. We must observe and evaluate it in all relevant cell types, at scale. We must fully understand its collective components, how it connects to both biology and to chemistry. We believe this logical exploration of underlying disease-driving function stands to revolutionize our approach to therapeutics.
In fact, we’ve already seen that we can influence the mechanobiology of cells to our advantage: Treatments like the bronchodilator Albuterol for asthma and drugs like beta-blockers for arrhythmia and hypertension work like expert choreographers, helping cells find their rhythm again and restoring the proper routine. These drugs work by regulating contractile function at the cellular level. Our challenge now is to replicate these successes faster, more safely, and more broadly across disease. To do so, we must industrialize mechanobiology.
Overcoming the Challenges: Charting a New Course in Mechanobiology
Technological barriers, complexity, a lack of high-throughput methods, and the interdisciplinary nature of mechanobiology have traditionally posed challenges to deciphering this cellular ballet.
However, with the recent convergent advances across multiple technological fields (miniaturization, automation, image processing, and gene-editing) and a growing appreciation for the pan-biological significance of mechanical function, we are poised to make significant strides.
We’ve already seen this function-focused approach to mechanobiology take shape among the various “heart-on-a-chip” models which capture the heart cell’s rhythmic contraction, an easy-to-follow periodic dance move. But as we’ve discussed, the dance of human mechanobiology is so much grander than just the heart, comprising a wide range of other ensembles, including slower, subtly contracting performers like smooth muscle cells or fibroblasts, which may take hours to complete their routines.
To realize the potential impact of a function-first approach to mechanobiology, we require a biology-invariant platform that can be tuned to any performance and used to scientifically study mechanical function in all cell types.
Tackling mechanobiology with FLECS: the world’s first comprehensive mechanomics platform
To properly parse mechanobiology and uncover the level of insight needed to have a realistic opportunity at successful translation in the clinic, we must enable access to mechanical cell function across a range of time scales in all human cell types. We are not merely thinking of experiments numbering in the 10s, 100s, 1000s, or even 10,000s. That would not be enough. The human genome contains some 20,000 genes but with redundancy and replicates, it may require 100,000 experiments to fully evaluate each gene in an arrayed format. Modern drug libraries contain 100,000s to millions of compounds requiring as many experiments to complete screening. And that’s only in one cell type. We think bigger. We aim to support 100,000s to millions of individual mechanobiological experiments performed rapidly for a given hypothesis or program.
Built with these critical practical considerations in mind from the beginning –ease-of-use, scalability, and throughput — we’ve built a universally applicable technology platform for studying the dance of human mechanobiology:
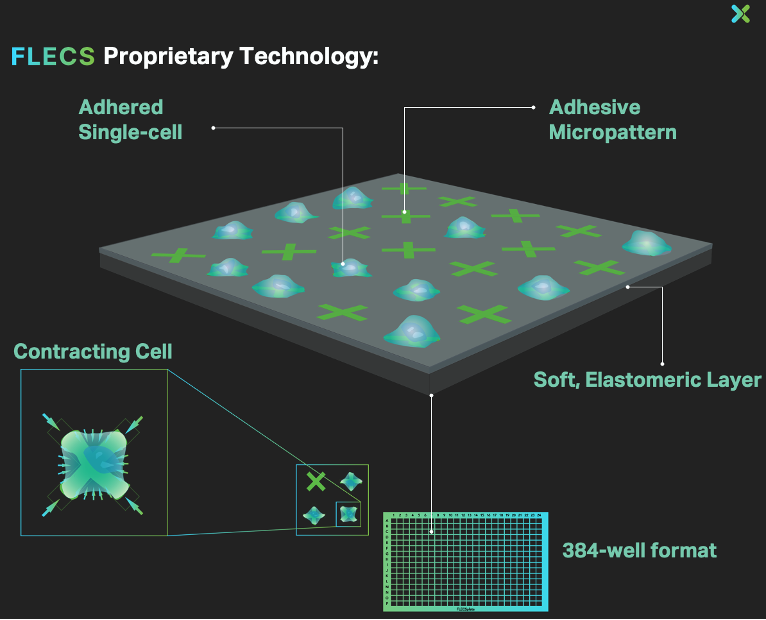
We built the FLECS Platform — an innovative technology representing a massive step-function evolution in our ability to functionally characterize mechanobiological function. (See: Nature Biomedical Engineer paper describing the platform).
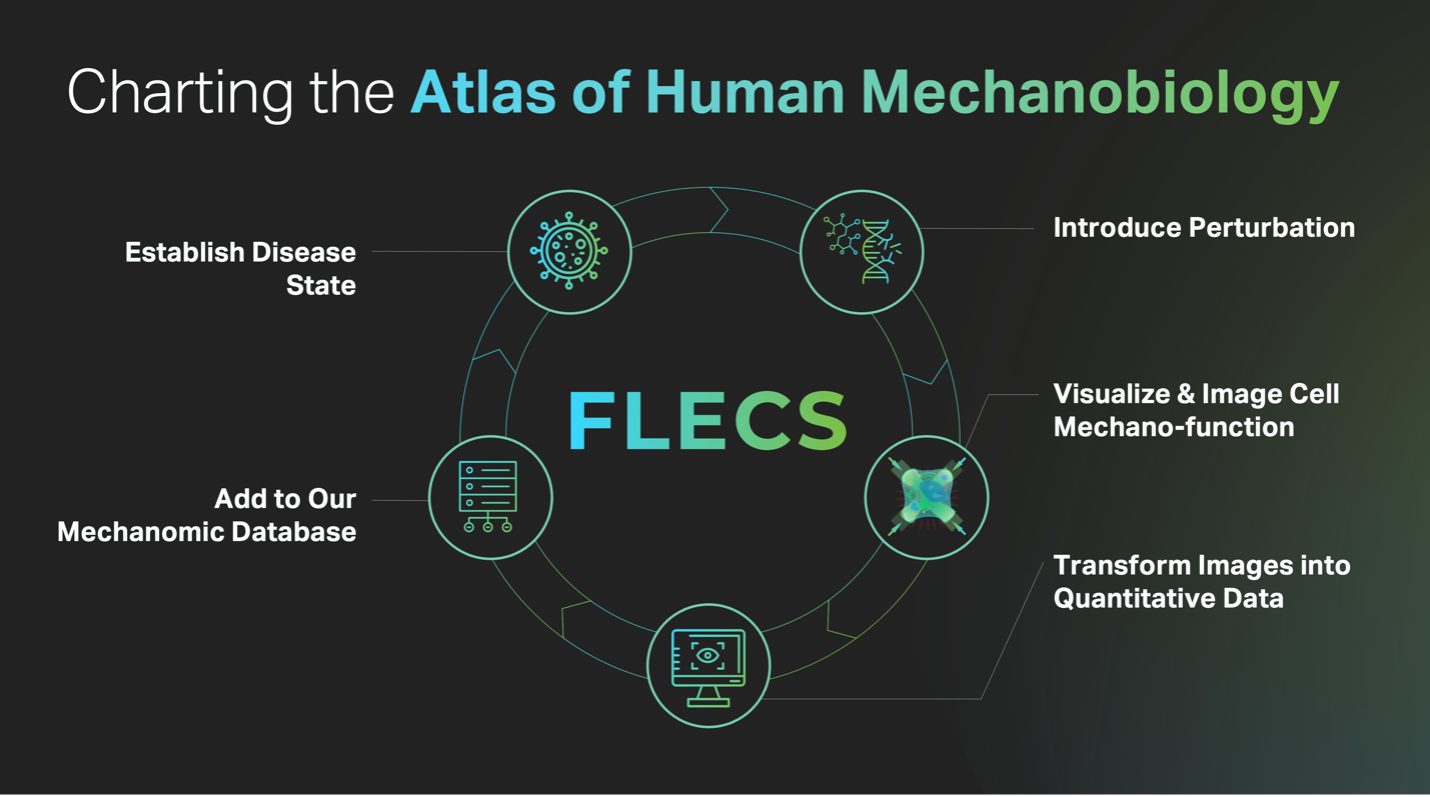
Charting the Atlas of Human Mechanobiology
Our FLECS platform provides Forcyte a unique platform advantage which enables us to run >15,000 mechanobiological experiments per screening day, each comprising 100s of single-cell data points, all with end-to-end automation. This platform advantage has thus given Forcyte an enormous data advantage representing what we believe to be the world’s largest specifically designed and highly curated mechanobiology data repository.
Through our efforts in mapping mechanobiology to chemistry (our “ChemFLECS” platform) we’ve screened >300,000 small molecule compounds across multiple unique primary human cell types, at both short-and long- term exposures. This data is interoperable reporting mechanical function along a common axis and serves as inherent counter-screening data when prioritizing hits for further development, while also building toward our overall database of mechanobiology. This effort has seeded our phenotypic pipeline with potent mechano-modulators of airway constriction, bladder spasms, and of TGFβ-driven myofibroblast contractility that are otherwise safe in terms not interfering with important contractile phenotypes in other tested tissue types.
Our work in mapping mechanobiology to biology (our “FuncFLECS” platform”) has already yielding functional contractility data from 3200 arrayed gene-silencing experiments corresponding to 800 unique kinase and phosphatase genes in multiple primary human cell types representing disease areas of high unmet need. This enabled us to link expression of these genes to their modulation of mechanical function in human cells, revealing new relationships that represent novel target hypothesis. We are now expanding our focus to screening the entire human genome and with the grand goal of identifying all the genes that modulate contractile cell function — something that’s previously been impossible.
These two parallel datasets in chemistry and biology combine to provide an even deeper level of insight at their intersection. The overlay of a results from targeted small molecule inhibitor library screen with results from the corresponding gene family screen offers orthogonal confirmation of the roles of specific genes in driving contractile function, providing further validation of potential new targets
Overall, our synergistic focus on function in both chemistry and in biology unlocks the best of both worlds of phenotypic and target-based approaches to drug discovery, providing Forcyte valuable opportunities both for partnering with external drug-developers and for capturing maximal value by pursuing program development internally.
Embracing the Dance: The Future of Mechanobiology
As we venture into this new era of drug discovery, we are poised to harness the full potential of mechanobiology, extending beyond simply observing the dance to actually choreographing it for the benefit of patients.
While the desire to access this critical mechanical function as an assay endpoint isn’t new (with academic-stage approaches scattered throughout the literature and even NIBR recently entering the mix with an approach aiming to model hypercontractility in asthma ), in drug discovery, execution and data quality are everything.
In this regard, no other technology comes close to the throughput, adaptability, cost-effectiveness, automation readiness, and quantitative resolution as FLECS. Our bodies are indeed mechanical systems and Forcyte stands tall as the undisputed technological leader in the space and is spearheading the movement to bring mechanobiology out of the shadows and into the forefront of cutting-edge drug discovery.
Stay tuned for more updates, perspectives and detailed info on how Forcyte is leveraging our FLECS Platform to re-imagine mechanobiology to make an impact.

